
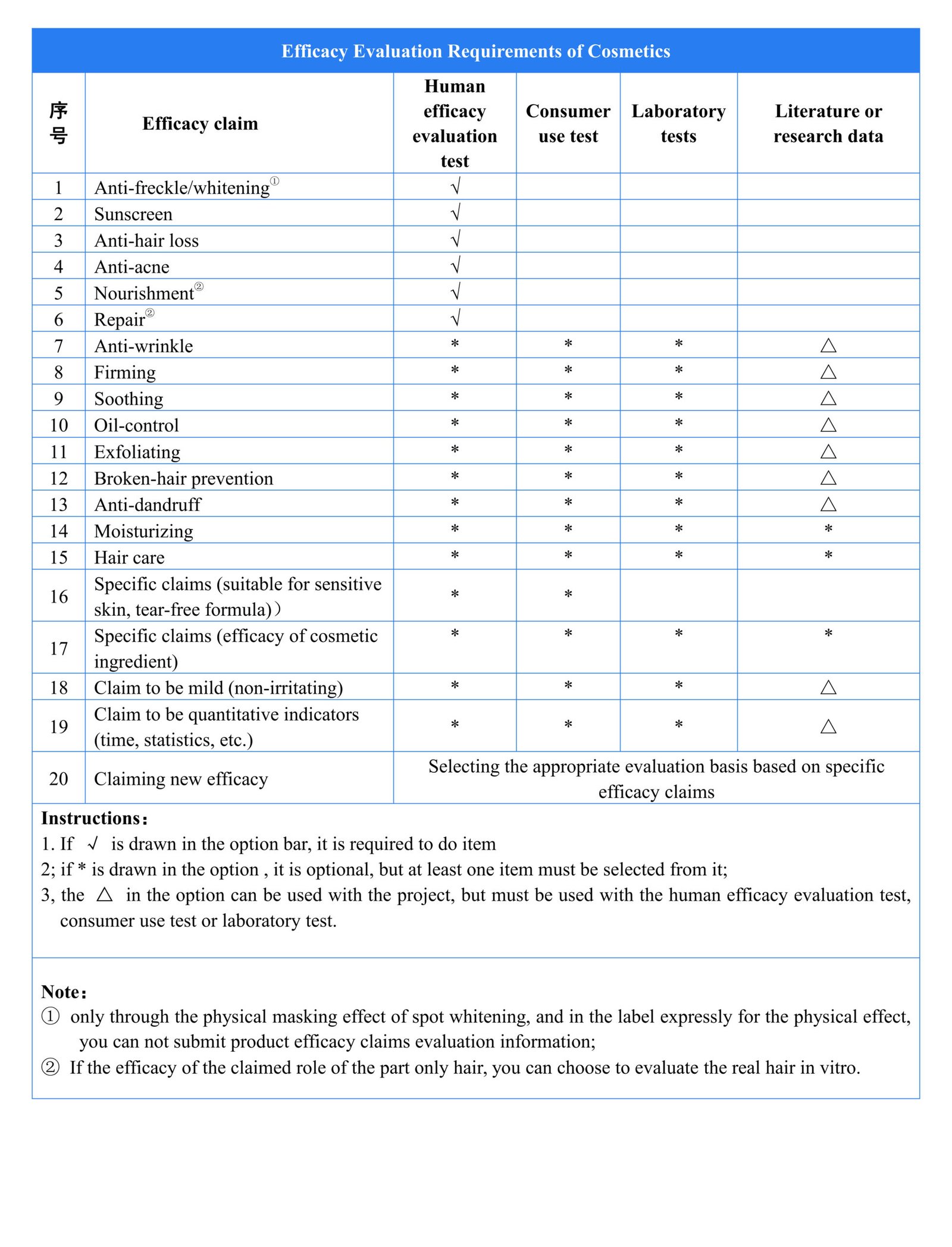
According to the Supervision and Administration Regulations (CSAR), the efficacy claims of cosmetics should have a sufficient scientific basis. Cosmetics registrant and filer should publish a summary of the literature, research data or product efficacy evaluation information on the efficacy claims to the NMPA designated website for public supervision.
Cosmetic registrant and filer should be responsible for the scientificity, authenticity, reliability and traceability of the submitted summary of the basis for the efficacy claims.
Copyright 2023 © All Right Reserved Design by Cosmetic Expert
We will contact you witthin 1 working day